Ofloxacin Residue Colloid Gold Rapid Detection Card Instruction Manual
Product Number: YB122D01K
Summary
Ofoxacin (OFL) is a third-generation quinolone antibacterial drug with broad-spectrum antibacterial activity and good bactericidal effect. It has good antibacterial effect on Staphylococcus, Streptococcus, Enterococcus, Gonococcus, Escherichia coli, Shigella, Enterobacter, Proteus, Haemophilus influenzae, Acinetobacter. It also has certain antibacterial effect on Pseudomonas aeruginosa and Chlamydia trachomatis. The national standard stipulates that the maximum residue limit of Ofloxacin in all food animals is 2 μg/kg. This product is suitable for on-site rapid detection of enterprises, testing institutions, supervision departments and other types.
Detection principle
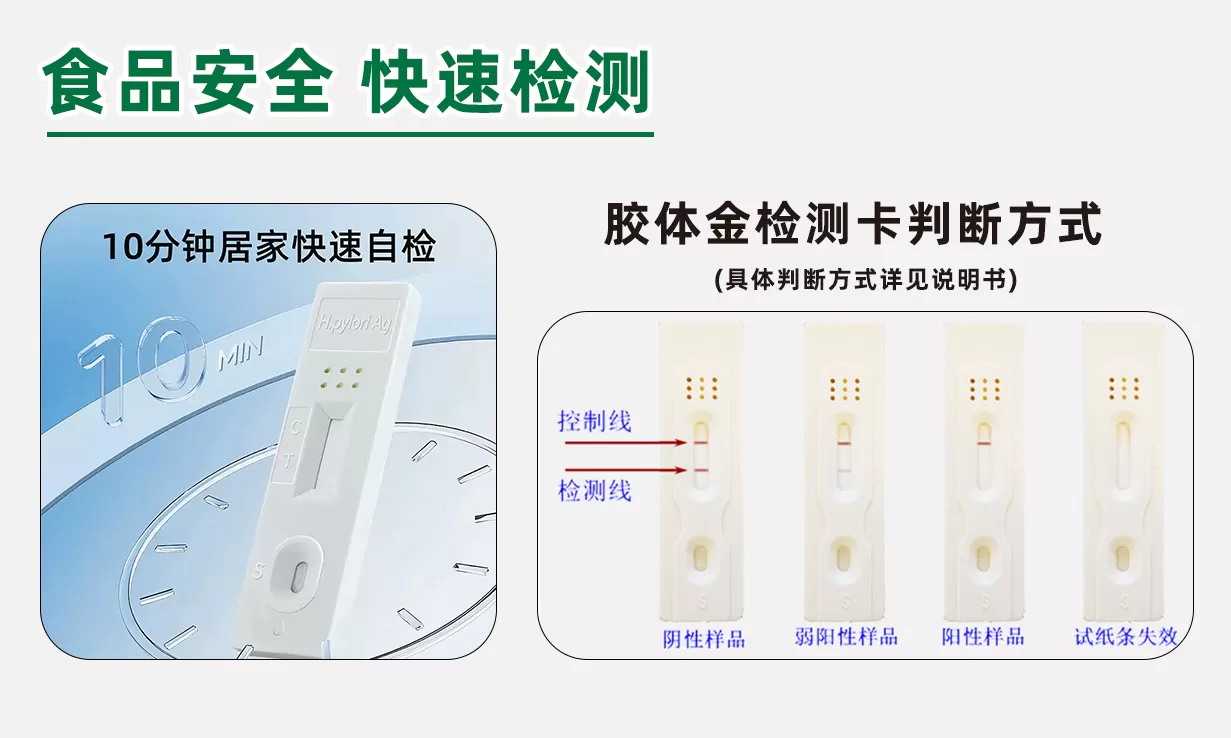
This product is made of the principle of competitive inhibition colloidal gold immunochromatography for the detection of ofloxacin residues in poultry egg samples. After the sample solution is dropped into the sample hole of the test card, the ofloxacin in the sample solution is combined with the gold standard antibody, thereby preventing the gold standard antibody from binding to the ofloxacin conjugate on the cellulose membrane. The judgment result is based on the color development depth of the T-line and the C-line.
Scope of application
This product is suitable for the qualitative detection of ofloxacin residues in poultry egg samples.
Note: For the type of test sample, refer to the national standard GB2763-2021.
detection limit
20 ppb (μg/kg)
Kit composition
serial number
Specifications
Composition
1 0 times/box
20 times/box
(1)
Test card (containing dropper, desiccant)
10 parts
20 parts
(2)
gold standard micropores
10 pieces/cylinder
20 pieces/cylinder
(3)
dilution
1 bottle
2 bottle
(4)
1 0.5 mL centrifuge tube
10 pieces
20 pieces (5)
manual
1 parts
1 parts
Precautions (1) Before each sample, the knife that shredded the sample needs to be cleaned to avoid cross-contamination. 117
(3) Please follow the test steps for testing. Do not touch the color-developing area of the test strip during operation to avoid direct sunlight and direct blowing of electric fans.
(4) Please use the sample as soon as possible after processing. If the time is too long, you need to re-process the sample for re-testing.
(5) The solution of the sample to be tested needs to be clarified, otherwise it will easily lead to abnormal phenomena such as inconspicuous color development, which will affect the judgment of the experimental results.
(6) Products that have expired or damaged aluminum foil bags cannot be used. Please use the test card immediately after unpacking.
(7) This product is a one-time product. Do not reuse or mix test cards from different batches.
(8) When a positive result appears, it is recommended to re-test. The test results of this product are for reference only. If you need confirmation, please refer to the relevant national standards and methods.
(9) During product evaluation, if the standard product needs to be tested directly, it needs to be prepared with the special dilution solution in the kit.
(10) Tap water, distilled water, purified water or deionized water cannot be used as a negative control.
Safety instructions
(1) The experiment needs to match the corresponding experimental equipment and wear the necessary experimental equipment (white clothes, gloves, masks, etc.).
(2) The detection kit needs to be stored in a place that is not easy for children to contact.
(3) All items used in the experiment should be properly disposed of after application.
(4) Keep the laboratory clean and the air flow of the experimental environment after the experiment.
(5) The experimental waste is collected separately, and it is recommended to dispose of it as medical waste.
(6) Do not eat the equipped reagents.
storage conditions and valid period
(1) Storage conditions: 2-30 ℃ protected from light, do not freeze.
(2) valid period: 12 months.
![]()