Instructions for Instruction of Sodium Dehydroacetate Rapid Test Card (Colloidal Gold Method)
1. Test Principle
Immunochromatographic Competitive Method. During detection, the sodium dehydroacetate in the treated sample binds to the sodium dehydroacetate gold-standard antibody conjugate at the gold-standard pad, thereby inhibiting the binding of the sodium dehydroacetate gold-standard conjugate to the sodium dehydroacetate antigen on the solid-phase carrier membrane. If the content of sodium dehydroacetate in the treated sample is greater than the corresponding detection limit, the color development of the T-line is weaker than or equal to the C-line, and vice versa.
2. Intended Use
The initial screening of fruit samples ensures that the content of sodium dehydroacetate is not higher than the national standard.
3. Cross-reaction and product performance
Detection limit: 2ppm
The test of adding 10ppm cyclamate, sodium saccharin, sodium benzoate was negative; the false positive rate of this product was 15%, and the false negative rate was 5%.
Fourth, the main components
Component name
20 parts/box
Component name
20 parts/box
Sodium dehydroacetate test card
20 parts
Sodium dehydroacetate special extract
2 Bottle
Instruction manual
1 parts
/
/
Fifth, storage conditions and valid period
Original packaging: 4-30 ° C Dry storage, valid period 12 months.
After opening
VI. Sample requirements
1. Avoid spoilage and deterioration of the sample;
2. Avoid large pieces of soil (which can be thrown off or dialed off with other clean items).
VII. Test method
Sample pre-treatment
1. Take 20-50g representative sample and chop it (less than 1 square centimeter). Weigh 10.05g sample in a 15ml centrifuge tube, add 5mL of sodium dehydroacetate special extract, cover, shake violently for 1 minute, let stand for 1 minute, the supernatant is the liquid to be tested 1;
2, take 750μL of sodium dehydroacetate special extract and add 250μL of the liquid to be tested 1 and mix well, which is the liquid to be tested.
8. Sample testing
1. Disassemble the test card and place it horizontally on a clean experimental table;
2, absorb 100 μL (about 3 drops) of the liquid to be tested and add it to the sample well for 6 minutes.
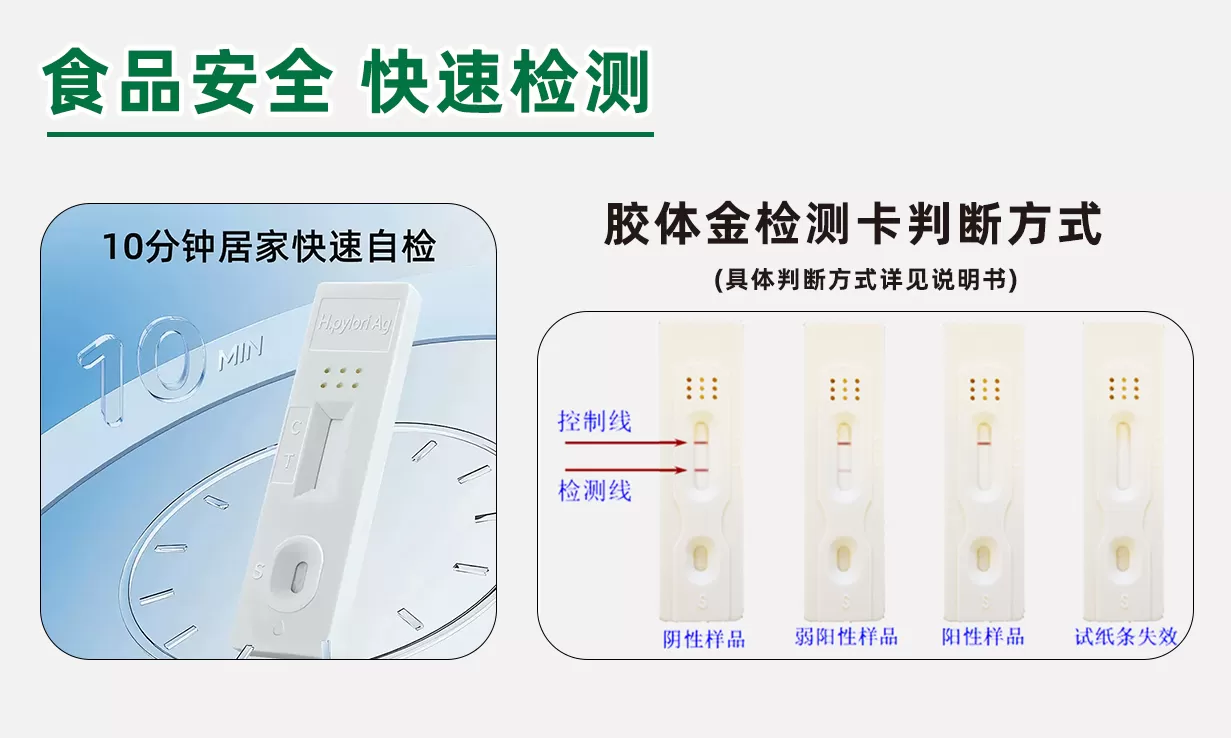
9. Interpretation of test results
Visual test:
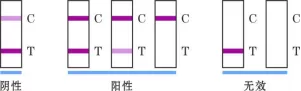
negative (not detected): T-line color > C-line color;
Positive (detected): T-line color C-line color; T-line does not develop color;
Invalid: C-line does not develop color and regardless of whether T-line develops color.
Instrument interpretation: For details, please refer to the instrument instruction manual
10. Precautions
1. This product is only for qualitative screening. If you need confirmation, please refer to the relevant national standard methods.
2. It is recommended to repeat the verification once when encountering positive samples.
3. Do not mix test cards and reagents from different batch numbers, and do not use kits that exceed the valid period.
4. The extracted sample is recommended to be used for the next test within 15 minutes.
5. After the detection reagent is taken out from the refrigerator, the temperature should be restored to room temperature before starting the test.
6. Before the test, it is recommended that the sample be fully stirred and mixed, so that the test results can more realistically reflect the actual drug residue of the sample.
7. The reagents involved in this product are safe and reliable, do not contain carcinogenic, highly toxic, flammable, explosive and highly corrosive reagents.
8. The reagents of this product are disposable products, and the waste after use should be treated as general chemicals.